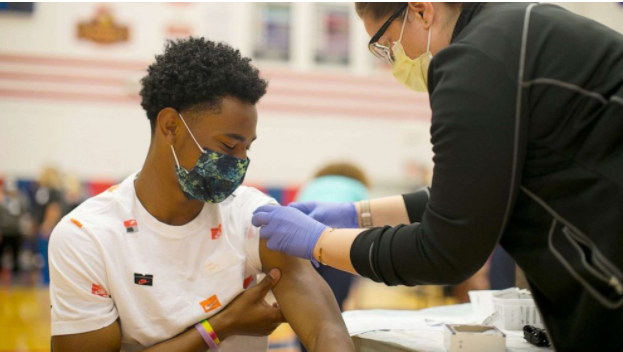
Over the weekend, the FDA approved the use of two malaria drugs for the treatment of COVID-19, under an “emergency use authorization.”
Chloroquine and hydroxychloroquine have been identified as potential COVID-19 treatments, which allows companies to donate these drugs to the Strategic National Stockpile. However, according to npr.org, “the FDA decision doesn’t reflect an official determination that the drugs work against the coronavirus.”
Despite this, since there are no treatments for the virus, many hospitals are already using these drugs on severely ill patients, according to nytimes.com.
Now, these drugs may not be used during clinical trials. Instead, doctors must now report on how the drugs were used, as well as report any harmful side effects.